ALL ALS is working to build a dataset to be used for ALS research! Whether you are an individual with ALS, family member, friend, or would just like to contribute to our research, there is a place for you to get involved with one of our studies!
Research into the natural history of ALS, including its earliest manifestations in asymptomatic ALS gene carriers, is a top priority. These research efforts are expected to result in knowledge that can lead to more informative, targeted, and personalized drug development, taking the field one step closer toward the goal of halting, repairing, and/or preventing ALS.
Who can participate?
You may not qualify to participate if you experience the following:
Accordion Content
Our aim is to make research accessible to all, not just those who can visit academic medical centers. Not only we will we remove barriers through new technology allowing for remote participation, this study will set the new standard for how we learn about ALS, and we cannot do that without understanding your unique experience of ALS.
By studying data from a wide spectrum of people, we can learn more about ALS and make future clinical trials better and more accessible.
Who can participate?
The study includes 1600 ALS patients (Cohort 1) and 450 control participants (Cohort 2).
Cohort 2- Must not have ALS or be known to be at risk of carrying a causative ALS gene mutation.
This may include spouses of ALS participants, family members of people with known ALS mutations who have tested negative for the family ALS causative gene mutation and other ALS gene mutations, and members of the general US population and others encountered at ALL ALS Sites.
You may not qualify to participate if you experience the following:
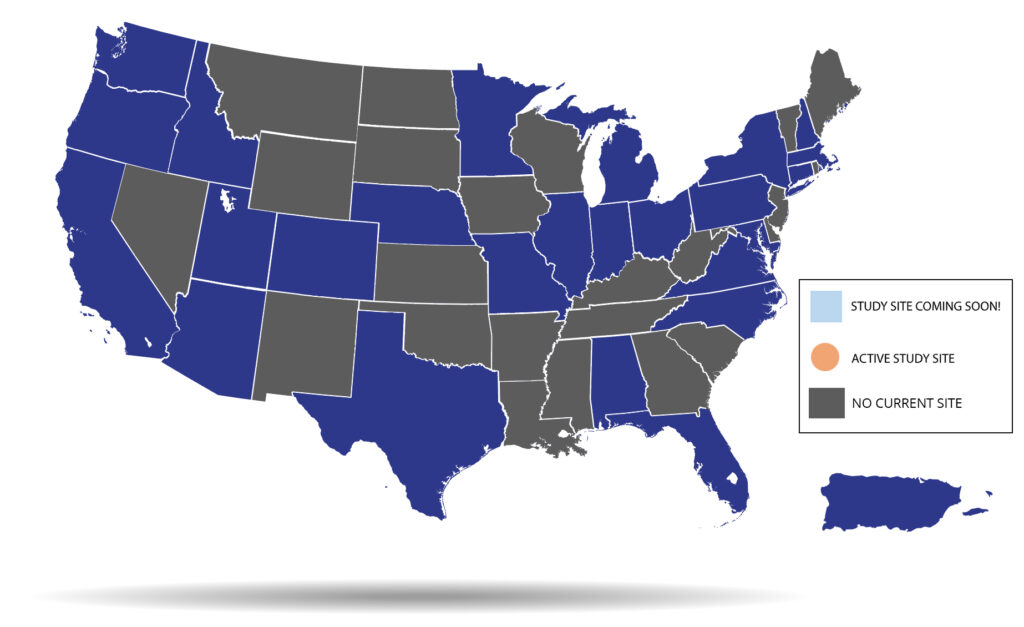
Study visits will be conducted during routine clinical visits or as stand-alone research visits, with either a combination of in-person and remote visits or entirely remote visits. Please see the map and site list below to find the study site closest to you!
Barrow Neurological Institute
Washington University:
Mass General:
Texas Neurology:
Ohio State University
University of Washington:
John Hopkins:
VCU (Virginia Commonwealth):
Hospital for Special Care:
Penn State Health:
University of Puerto Rico:
Dartmouth-Hitchcock Clinic:
University of Alabama at Birmingham:
University of Michigan:
University of Nebraska Medical Center:
University of Colorado Anschutz Medical Campus:
Columbia University:
Saint Alphonsus Regional Medical Center:
Georgetown University:
UC Irvine:
University of California, San Francisco:
University of Utah:
Henry Ford Health:
Indiana University :
Temple University:
Mayo Clinic:
Providence ALS center:
UC San Diego Health:
NIH/NINDS Clinical Research:
Northwestern University:
University of Minnesota:
University of Pennsylvania:
Nova Southeastern University:
| Site Name | City | State/Territory | Site Investigator(s) | Phone Number | Contact(s) | Actively Enrolling? |
|---|---|---|---|---|---|---|
| Barrow Neurological Institute | Phoenix | AZ | Shafeeq Ladha, MD Kerry Knievel, DO | 602-406-7773 | Christopher Shiver: fulton.research@commonspirit.org | YES |
| CHALS-CCT, University of Puerto Rico, Medical Sciences Campus | San Juan | PR | Valerie Wojna Muñiz, MD, FAAN Brenda Deliz Roldan, MD | 787-767-9194, 787-209-4315 | Frances Aponte: frances.aponte2@upr.edu | YES |
| Columbia University | New York | NY | Neil Shneider, MD, PhD | 212-305-6788 | Ben Hoover: alsresearch@cumc.columbia.edu | YES |
| Dartmouth-Hitchcock Clinic | Lebanon | NH | Mark Garret, MD | 603-650-6496 | Gina Kersey: neuroresearch@hitchcock.org | YES |
| Duke University | Durham | NC | Xiaoyan Li, MD, PhD | alsresearch@dm.duke.edu | YES | |
| Georgetown University | Washington | DC | Brent Harris, MD, PhD | 202-784-0672 | Cassandra Holmes: cassie.holmes@georgetown.edu | YES |
| Henry Ford Health | Detroit | MI | Ximena Arcila-Londono, MD | 313-916-3359 | Maria Stotland: mstotla1@hfhs.org | YES |
| Hospital for Special Care | New Britain | CT | Kevin Felice, DO | 860-827-1958 Ext# 5992 | Sabine Lebel-Hardenack: shardenack@hfsc.org | YES |
| Indiana University | Indianapolis | IN | Cynthia Bodkin, MD | ASSESS: 317-963-7382; PREVENT: 317-963-7455 | ASSESS: Lisa Grinstead: lgrinste@iu.edu; PREVENT: Patti Hogan: hoganpr@iu.edu | YES |
| John Hopkins | Baltimore | MD | Nicholas Maragakis, MD | 410-502-6597 | Delayna Willie: dwillie2@jh.edu | YES |
| Mass General Hospital | Boston | MA | James Berry, MD | ASSESS: 617-643-9550; PREVENT: 617-724-9196 | ASSESS: Miranda Duncan, PREVENT: Anika Allen: mghassessals@mgb.org, mghpreventallals@mgb.org | YES |
| Mayo Clinic | Jacksonville | FL | Bjorn Oskarsson, MD | 904-953 3730 | Jany Dagher: dagher.jany@mayo.edu, Jeffery Gainer: gainer.jeffery@mayo.edu | YES |
| NIH/NINDS | Bethesda | MD | Justin Kwan, MD | 301-451-1229 | Katelyn Porter: katelyn.porter@nih.gov | YES |
| Northwestern University | Chicago | IL | Senda Ajroud-Driss, MD | (312) 503-5166 | Aeryn Hopwood: aeryn.hopwood@northwestern.edu | YES |
| Nova Southeastern University | Fort Lauderdale | FL | Eduardo Locatelli MD | (954) 262-6387 | Donovan Mott: donovan.mott@nova.edu | Yes |
| Ohio State University | Columbus | OH | Stephen Kolb, MD, PhD | 614-685-5815 | alsresearch@osumc.edu | YES |
| Penn State Health | Hershey | PA | Zachary Simmons, MD | 717-531-8257 | Michele Hare: nervemuscle@pennstatehealth.psu.edu | YES |
| Providence ALS Center | Portland | OR | Nicholas Olney, MD | 503-215-8580 | Providence Neurological Specialties - East | YES |
| Saint Alphonsus Regional Medical Center | Boise | ID | Jackie Whitesell, MD | 208-367-7397 | Helena Snider, Westin Payne: neuro.research@saintalphonsus.org | YES |
| Temple University | Philadelphia | PA | Terry Heiman-Patterson, MD | 267-694-0573 | John Furey: john.furey0001@temple.edu | YES |
| Texas Neurology | Dallas | TX | Daragh Heitzman, MD, FAAN | 214-827-3610 | Haley Rucker: hrucker@texasneurology.com; Reham Azab: razab@texasneurology.com | YES |
| University of Alabama | Birmingham | AL | Nan Jiang, MD | 205-975-0445 | Melanie Benge: melaniebenge@uabmc.edu | YES |
| University of California, Irvine | Irvine | CA | Namita Goyal, MD | 714-456-3947 | Rosa Gonzalez: rosaig1@hs.uci.edu | YES |
| University of California, Los Angeles | Los Angeles | CA | Martina Wiedau, MD | NO | ||
| University of California, San Diego Health | La Jolla | CA | John Ravits, MD | 858-246-2325 | Gil Gutierrez: grg005@health.ucsd.edu | YES |
| University of California, San Francisco | San Francisco | CA | Laura Rosow, MD | 415-353-2959 | Hannah George: hannah.george@ucsf.edu | YES |
| University of Colorado Anschutz Medical Campus | Aurora | CO | Laura Foster, MD | 303-724-4644 | Alexis Shepardson: neuroresearch@cuanschutz.edu | YES |
| University of Michigan | Ann Arbor | MI | Eva Feldman, MD , PhD | 734-936-8781 | Caroline Piecuch: carolinp@med.umich.edu | YES |
| University of Minnesota | Minneapolis | MN | David Walk, MD | 612-624-9987 | Julia Munoz: munoz156@umn.edu | YES |
| University of Nebraska Medical Center | Omaha | NE | Joseph Americo Fernandes, MD, FAAN | 402 552-6241 | Nathan McKain: nmckain@unmc.edu | YES |
| University of Pennsylvania | Philadelphia | PA | Colin Quinn, MD | 215-615-0550 | Matthew Burst: matthew.burst@pennmedicine.upenn.edu | NO |
| University of Utah | Salt Lake City | UT | Mark Bromberg, MD, PhD; | 801-581-7437 | Scott Redlin: scott.redlin@utah.edu | YES |
| University of Washington | Seattle | WA | Michael Weiss, MD | 206-543-0454 | Lila Brisk: lbrisk@uw.edu | YES |
| Vanderbilt University Medical Center | Nashville | TN | Amanda Peltier, MD | NO | ||
| Virginia Commonwealth University | Richmond | VA | Kelly Gwathmey, MD | 315-651-9378 | Brianna Schibley-Laird: brianna.schibleylaird@vcuhealth.org | YES |
| Washington University | St. Louis | MO | Timothy Miller, MD, PhD | 844-257-2273 | Jesse Markway: als@wustl.edu | YES |
The ALL ALS Consortium and all related studies are funded by the National Institutes of Health (NIH), Grant # 1OT2NS136938 and 1OT2NS136939.
Funded by the NIH/NINDS, the Access for ALL in ALS Consortium (ALL ALS), is a community of two coordination centers and 35 research sites across the United States, conducting a combined longitudinal natural history study and biomarker collection study for ALS.