Research into the natural history of ALS, including its earliest manifestations in asymptomatic ALS gene carriers, is a top priority. These research efforts are expected to result in knowledge that can lead to more informative, targeted, and personalized drug development, taking the field one step closer toward the goal of halting, repairing, and/or preventing ALS.



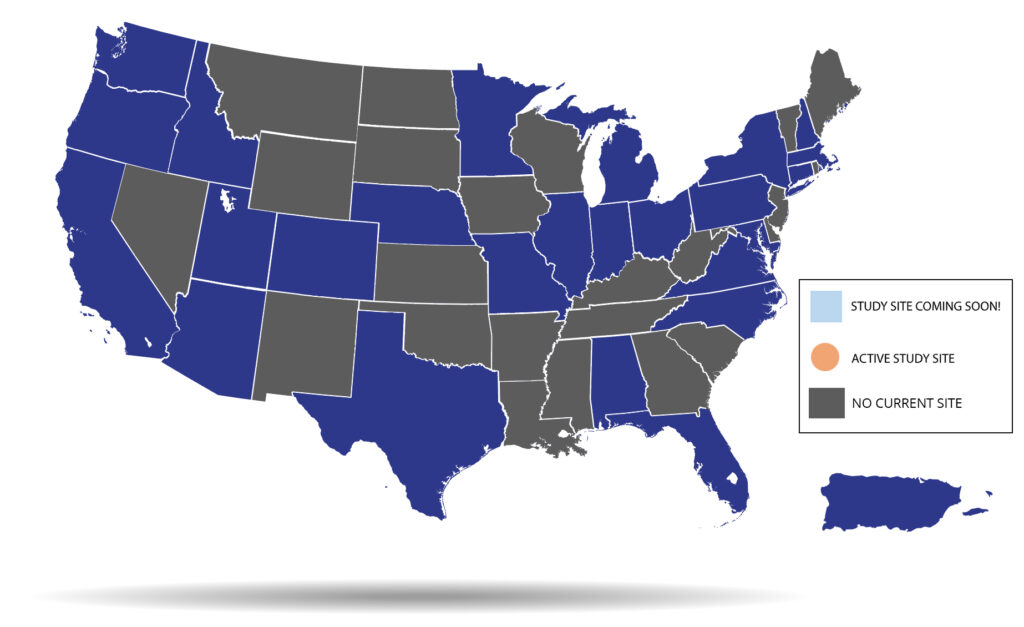
Study visits will be conducted during routine clinical visits or as stand-alone research visits, with either a combination of in-person and remote visits or entirely remote visits. Please see the map and site list below to find the study site closest to you!
Barrow Neurological Institute
Washington University:
Mass General:
Texas Neurology:
Ohio State University
University of Washington:
John Hopkins:
VCU (Virginia Commonwealth):
Hospital for Special Care:
Penn State Health:
University of Puerto Rico:
Dartmouth-Hitchcock Clinic:
University of Alabama at Birmingham:
University of Michigan:
University of Nebraska Medical Center:
University of Colorado Anschutz Medical Campus:
Columbia University:
Saint Alphonsus Regional Medical Center:
Georgetown University:
UC Irvine:
University of California, San Francisco:
University of Utah:
Henry Ford Health:
Indiana University :
Temple University:
Mayo Clinic:
Providence ALS center:
UC San Diego Health:
NIH/NINDS Clinical Research:
Northwestern University:
University of Minnesota:
University of Pennsylvania:
Nova Southeastern University:
| Site Name | City | State/Territory | Contact | Phone Number | Primary SC Email Address | Actively Enrolling? |
|---|---|---|---|---|---|---|
| Barrow Neurological Institute | Phoenix | AZ | Christopher Shiver | 602-406-7773 | fulton.research@dignityhealth.org | Yes |
| CHALS-CCT, University of Puerto Rico, Medical Sciences Campus | San Juan | PR | Frances Aponte | 787-767-9194 | frances.aponte2@upr.edu | Yes |
| Dartmouth-Hitchcock Clinic | Lebanon | NH | Kathleen Sullivan | 603-650-6496 | neuroresearch@hitchcock.org | Yes |
| Duke | Durham | NC | Emma Ward | 919-600-3626 | emma.ward@duke.edu | Yes |
| Hospital for Special Care | New Britain | CT | Sabine Lebel-Hardenack | (860)827-1958 ext# 5992 | SHardenack@HFSC.org | Yes |
| Johns Hopkins | Baltimore | MD | Delayna Willie | 410-502-6597 | dwillie2@jh.edu | Yes |
| Mayo Clinic | Jacksonville | FL | Jany Dagher, Jeffery Gainer | (904) 953 3730 | dagher.jany@mayo.edu gainer.jeffery@mayo.edu | Yes |
| Northwestern University | Chicago | IL | Anirudh Muralidharan Aeryn Hopwood | Anirudh: (312) 503-6823; Aeryn: (312) 503-5166 | anirudh.muralidharan@northwestern.edu | Yes |
| Penn State Health | Hershey | PA | Michele Hare | 717-531-8257 | nervemuscle@pennstatehealth.psu.edu | Yes |
| Providence ALS center | Portland | OR | Providence Neurological Specialties-East | (971) 231-8245 | Yes | |
| Saint Alphonsus Regional Medical Center | Boise | ID | Helena Snider, Westin Payne | 208-367-7397 | neuro.research@saintalphonsus.org | Yes |
| Texas Neurology | Dallas | TX | Haley Rucker | 214-827-3610 | hrucker@texasneurology.com | Yes |
| UC San Diego Health | La Jolla | CA | Gil Gutierrez | 858-246-2325 | grg005@health.ucsd.edu | Yes |
| University of Alabama at Birmingham | Birmingham | AL | Melanie Benge | 205-975-0445 | melaniebenge@uabmc.edu | Yes |
| University of California, San Francisco | San Francisco | CA | Hannah George | 415-353-2959 | hannah.george@ucsf.edu | Yes |
| University of Colorado Anschutz Medical Campus | Aurora | CO | Alexis Shepardson | 303-724-4644 | neuroresearch@cuanschutz.edu | Yes |
| University of Michigan | Ann Arbor | MI | Caroline Piecuch | 734-936-8781 | carolinp@med.umich.edu | Yes |
| University of Nebraska Medical Center | Omaha | NE | Nathan McKain | 402 552-6241 | nmckain@unmc.edu | Yes |
| University of Washington | Seattle | WA | Kinsey Chapman | 206-543-0081 | kinseyc@uw.edu | Yes |
| Virginia Commonwealth University | Richmond | VA | Brianna Schibley-Laird | 315-651-9378 | brianna.schibleylaird@vcuhealth.org | Yes |
| Washington University | St. Louis | MO | Jesse Markway | 1-844-257-2273 | als@wustl.edu | Yes |
| Mass General | Boston | MA | Courtney Uek | 617-724-0783 | cuek@mgb.org | Yes |
| Columbia University | New York | NY | Ben Hoover | 212-305-6788 | alsresearch@cumc.columbia.edu | Yes |
| Ohio State University | Columbus | OH | Carly Failor | 614-293-4973 | alsresearch@osumc.edu | Yes |
| UC Irvine | Irvine | CA | Rosa Gonzalez | 714-456-6191 | rosaig1@hs.uci.edu | Yes |
| University of Utah | Salt Lake City | UT | Scott Redlin | 801-581-7437 | scott.redlin@utah.edu | Yes |
| University of Minnesota | Minneapolis | MN | Julia Munoz | 612-624-9989 | munoz156@umn.edu | Yes |
| Indiana University | Indianapolis | IN | Angela Micheels | 317-963-7385 | amicheel@iu.edu | Yes |
| Temple University | Philidelphia | PA | John Furey | 267-694-0573 | john.furey0001@temple.edu/ | Yes |
| Georgetown University | Washington | DC | Arthur Zimmer | 301-956-6489 | az642@georgetown.edu | Yes |
| NIH/NINDS Clinical Research | Bethesda | MD | Katelyn Porter | 301-451-1229 | katelyn.porter@nih.gov | Yes |
| Henry Ford Health | Detroit | MI | Maria Stotland | 313-916-3359 | mstotla1@hfhs.org | Yes |
| Nova Southeastern University | Fort Lauderdale | FL |
The ALL ALS Consortium and all related studies are funded by the National Institutes of Health (NIH), Grant # 1OT2NS136938 and 1OT2NS136939.